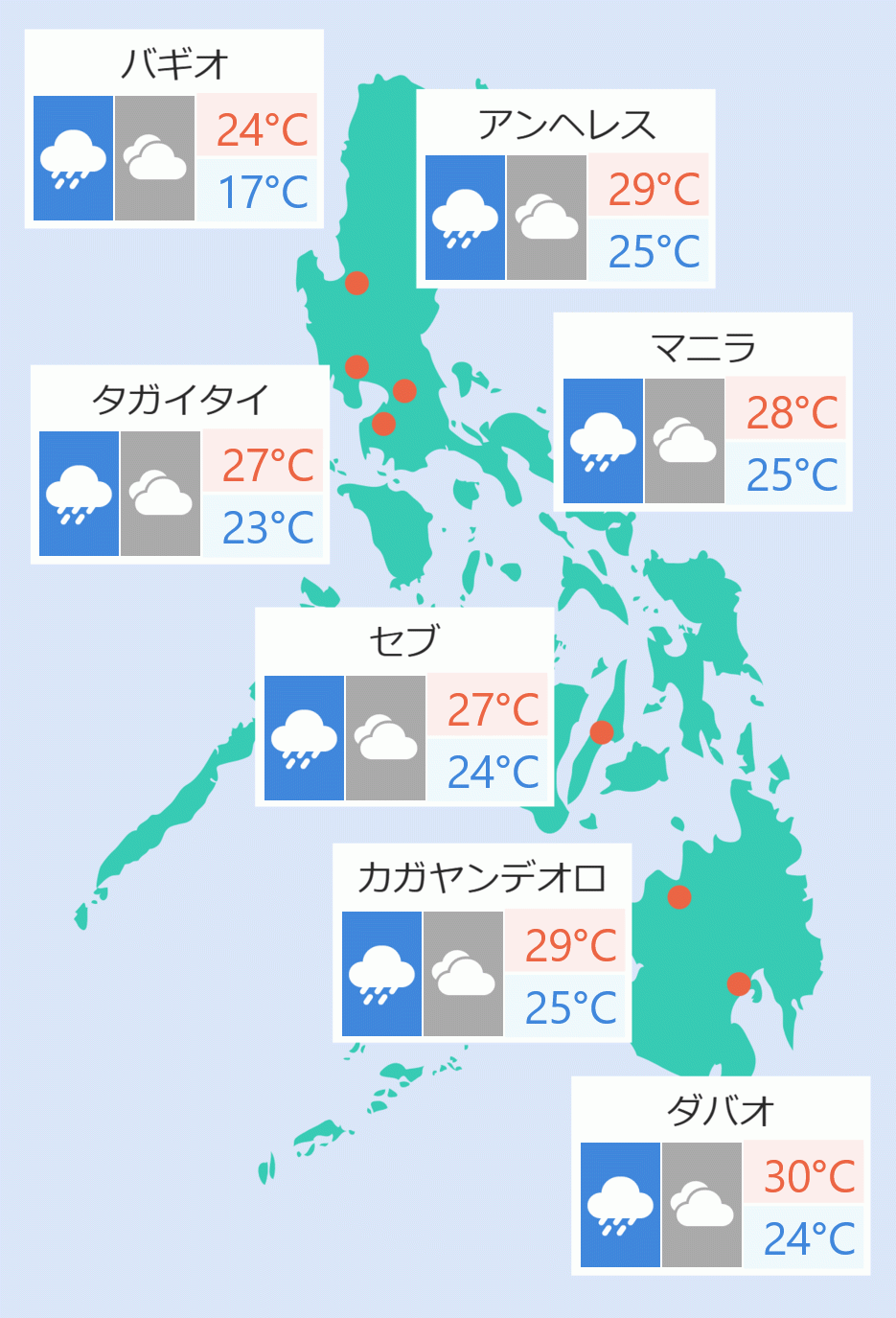
The Food and Drug Administration (FDA) has approved the emergency use authorization (EUA) for the oral COVID-19 drug Molnarz, its head announced Thursday.
Molnarz is the brand name of Molnupiravir, which is developed by Merck and Co.
FDA head Rolando Eric Domingo told the ''Laging Handa'' virtual public forum that Molnarz will be given for patients with mild to moderate COVID-19.
Molnarz should be given twice a day for five days immediately after one is diagnosed with COVID-19, said Domingo.
'' It should be given as soon as possible after diagnosis and within the first five days after the onset of symptoms,'' said Domingo. DMS





 English
English